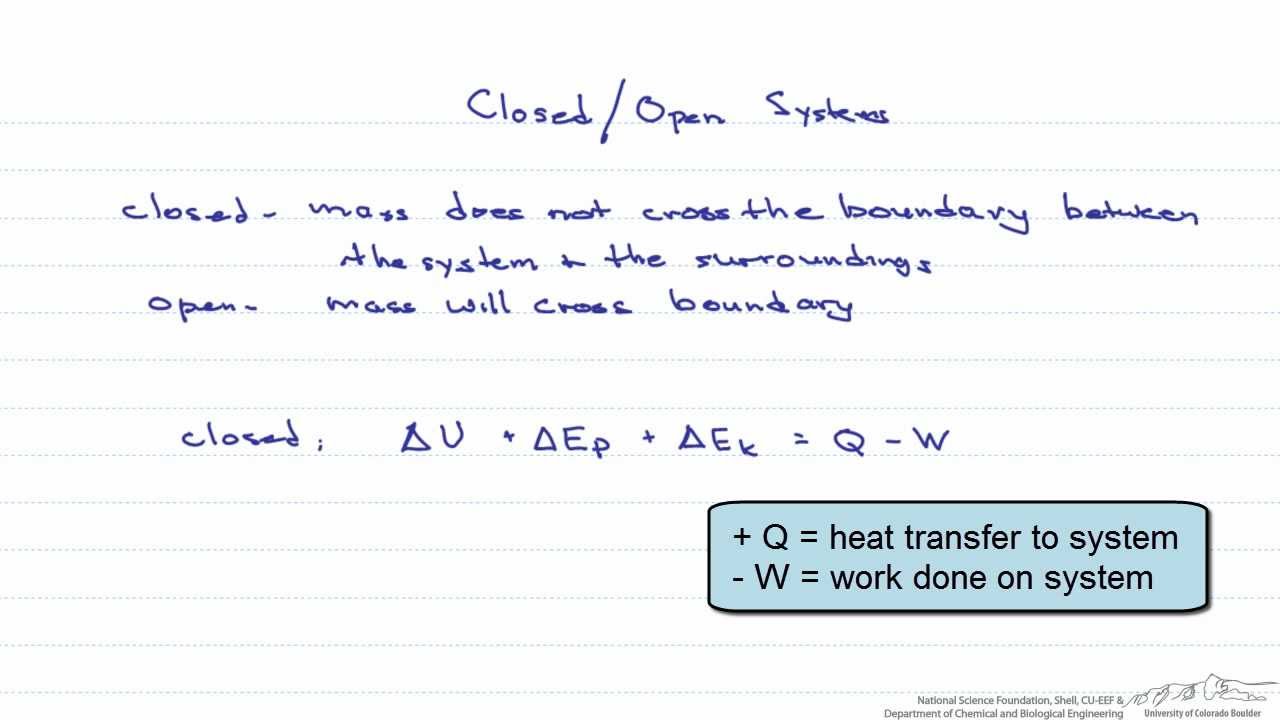
Closed System Energy Balance
Closed system energy balance. General balance equation is. In an open system the mass that. General balance equation is.
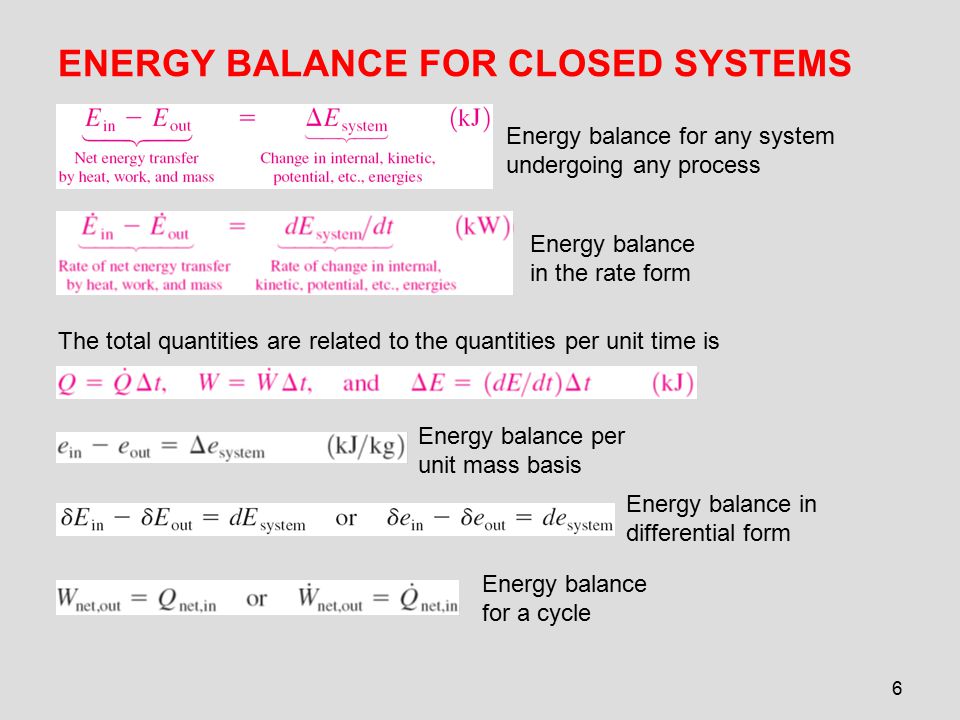
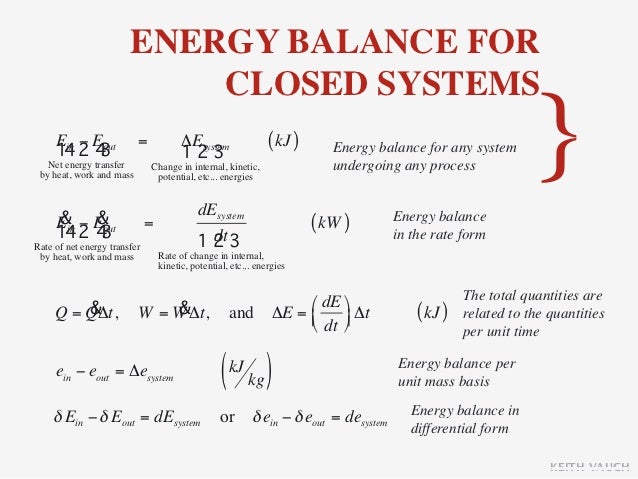
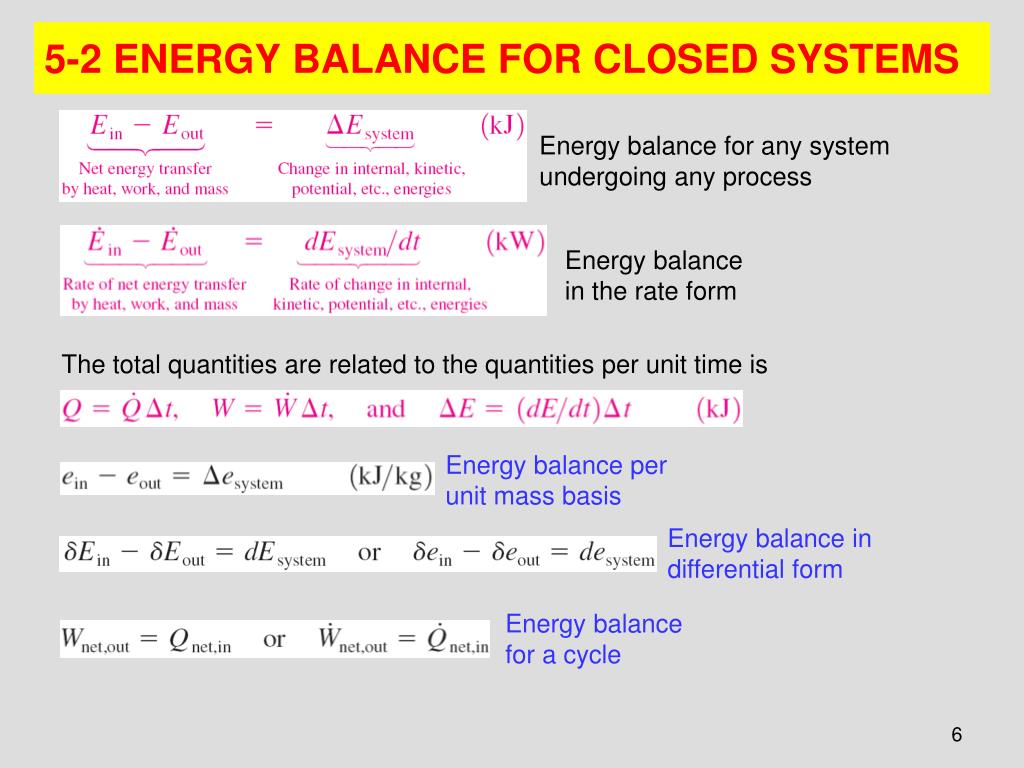
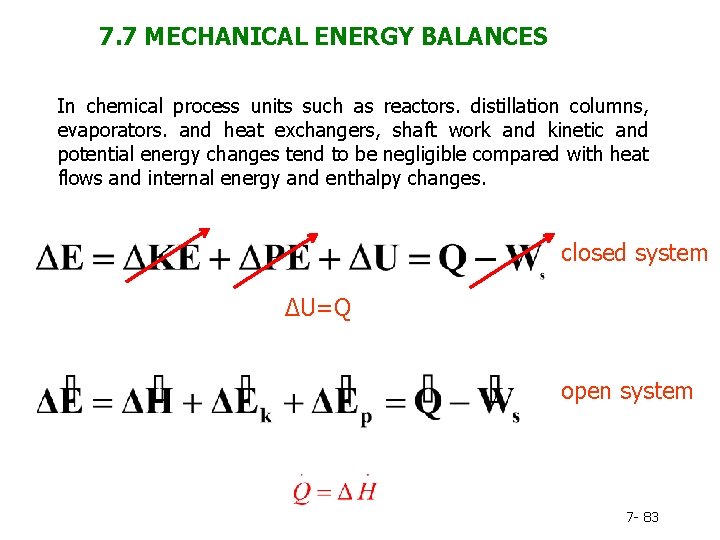
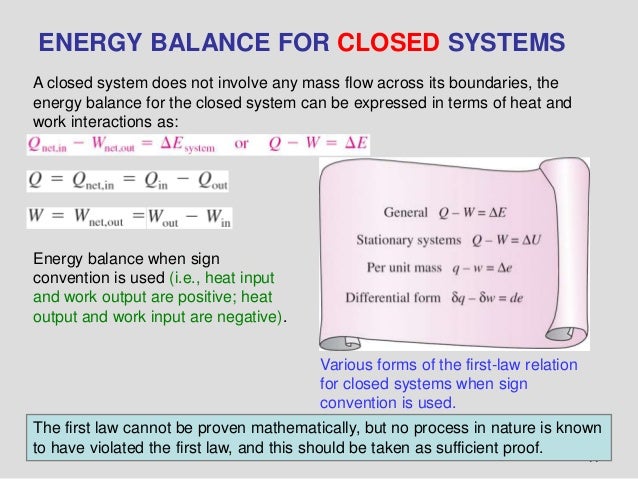
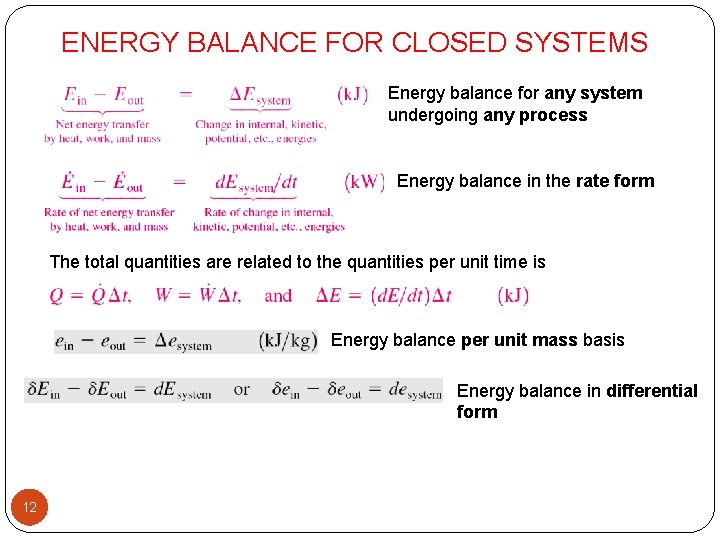
The exergy balance for a closed system is developed by combining the closed system energy and entropy balances. ENERGY BALANCE FOR CLOSED SYSTEMS Energy balance for any system undergoing any process Energy balance in the rate form The total quantities are related to the quantities per unit time is Energy balance per unit mass basis Energy balance in differential form Energy balance for a cycle. System Definitions and Application.
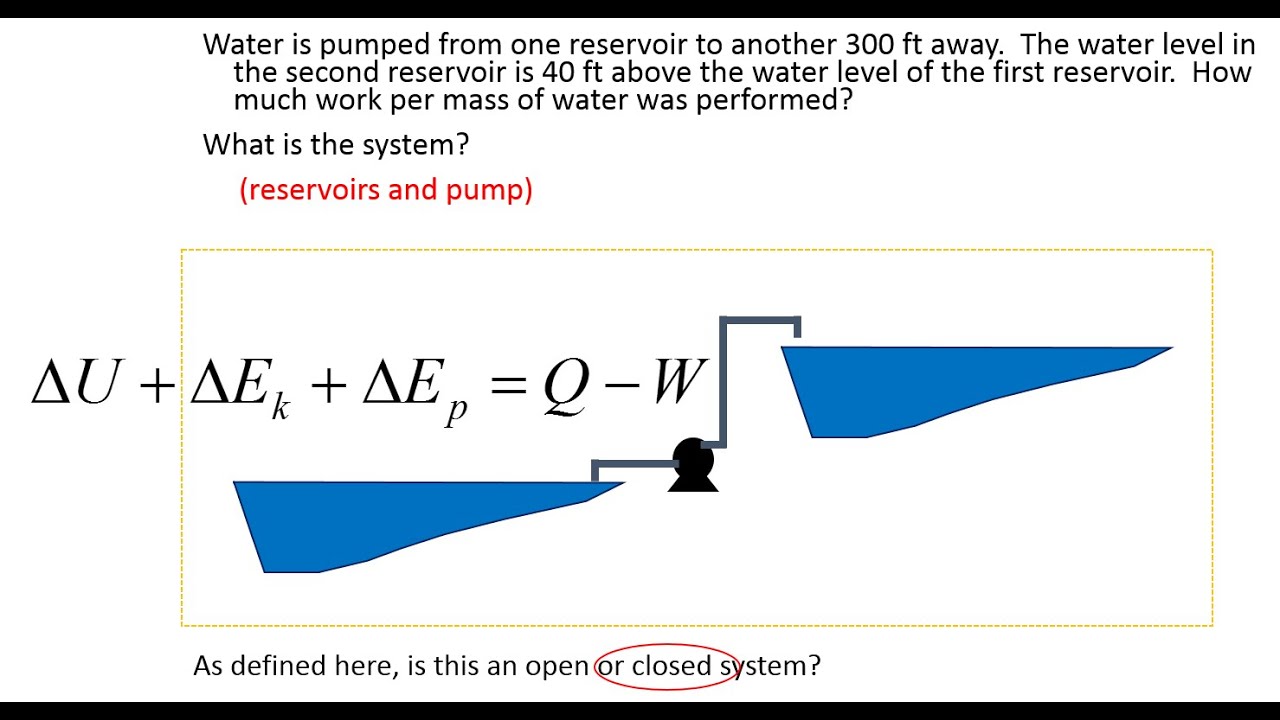
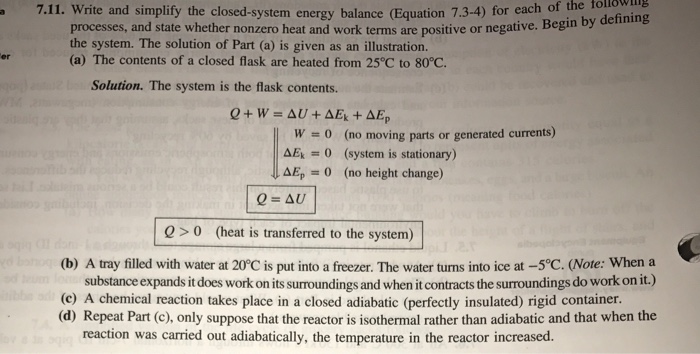
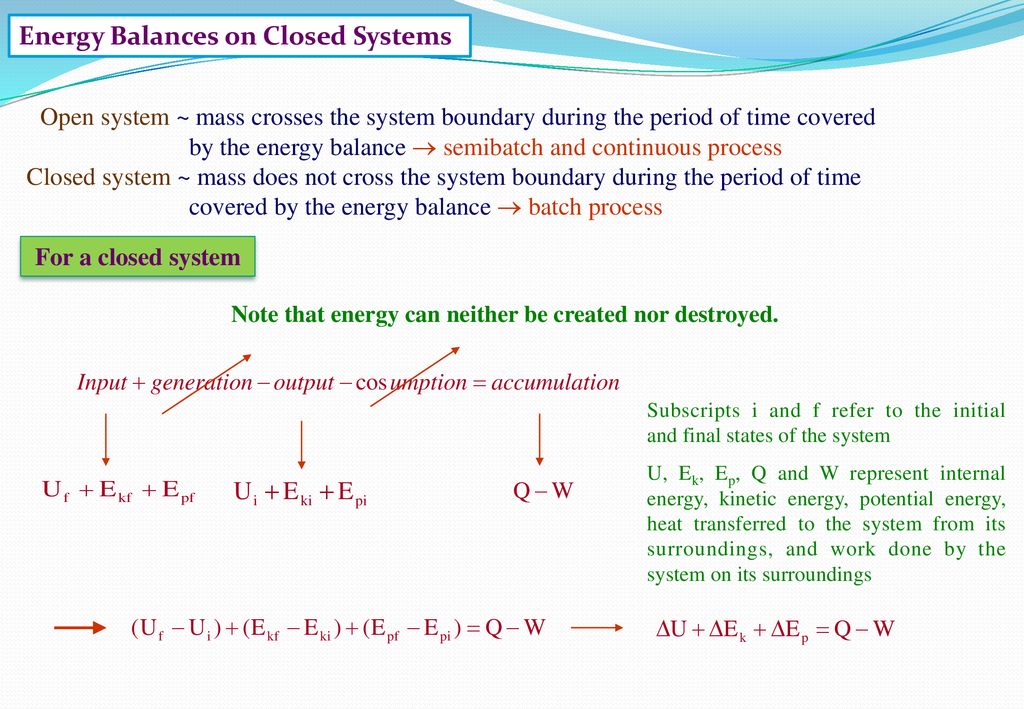
ΔE ΔKEΔP E ΔU Q W. Q is negative when system rejects heat. Q W U E E U E E f kf pf.
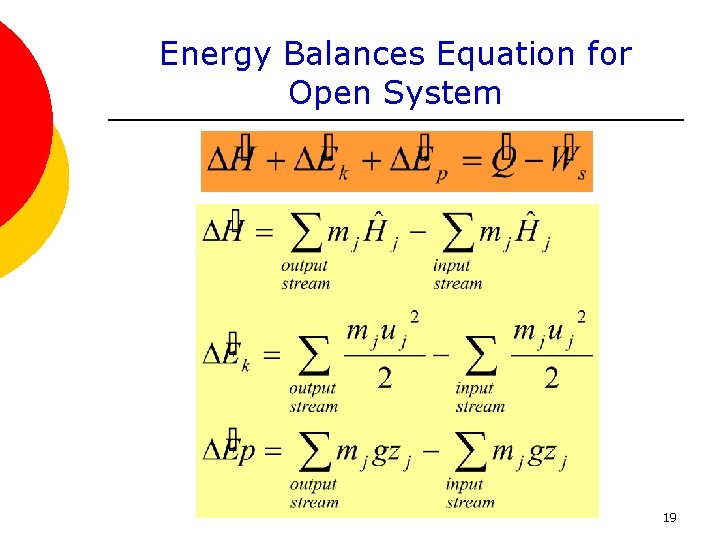
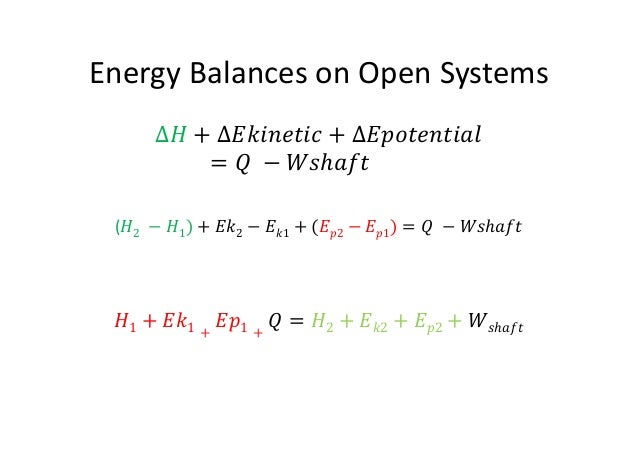
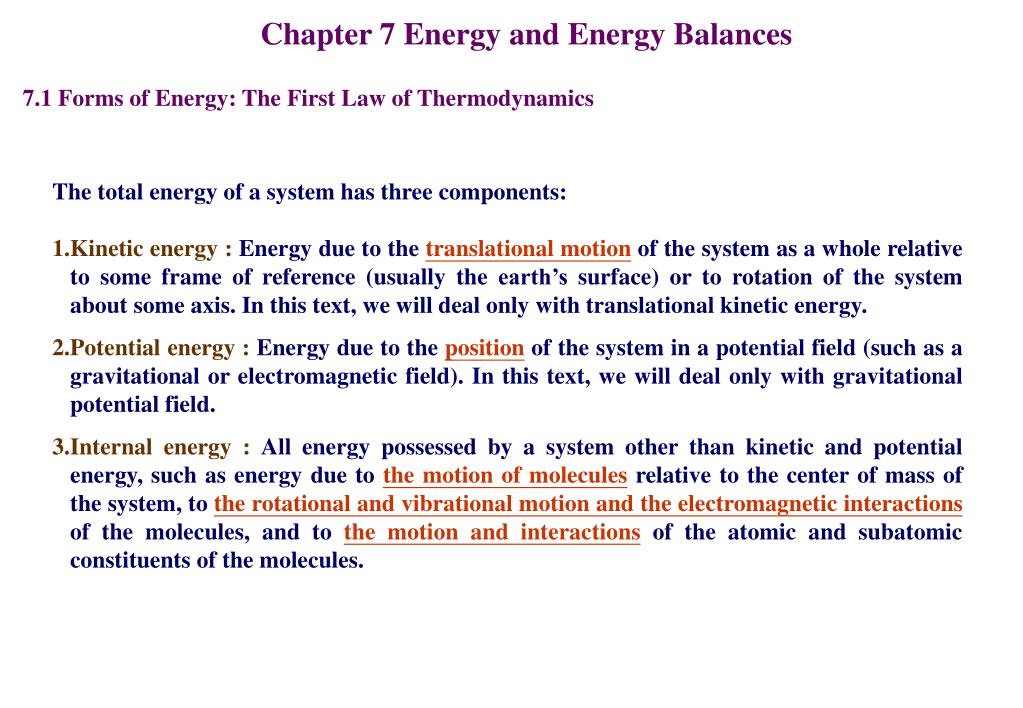
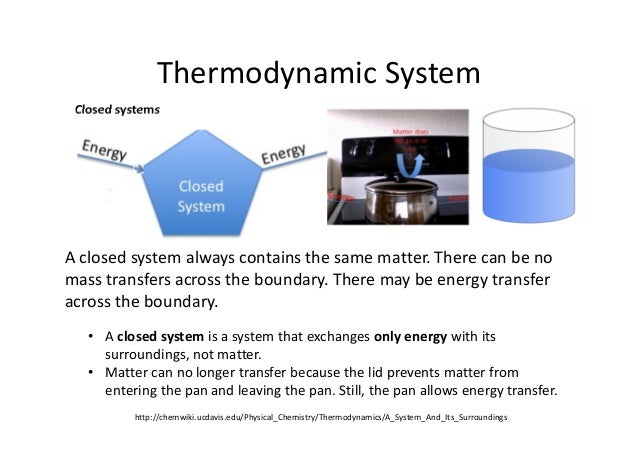
Energy is transferred between the system and the surroundings in the form of heat and work resulting in a change of total energy of the system. Closed and Open Systems. In open systems fluid particles move from the inlet to the outlet and leave the system.
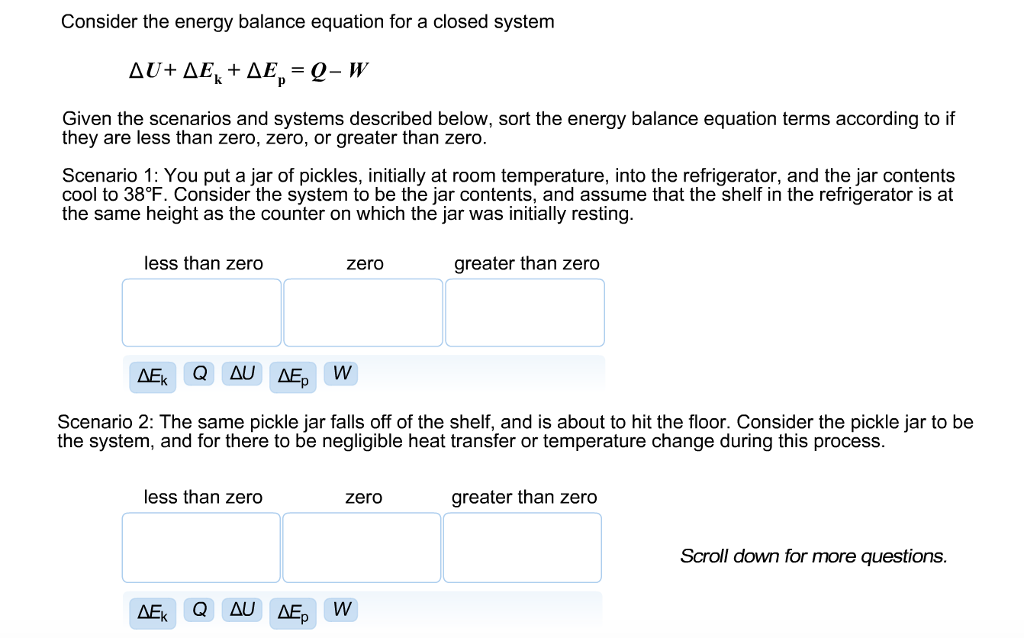
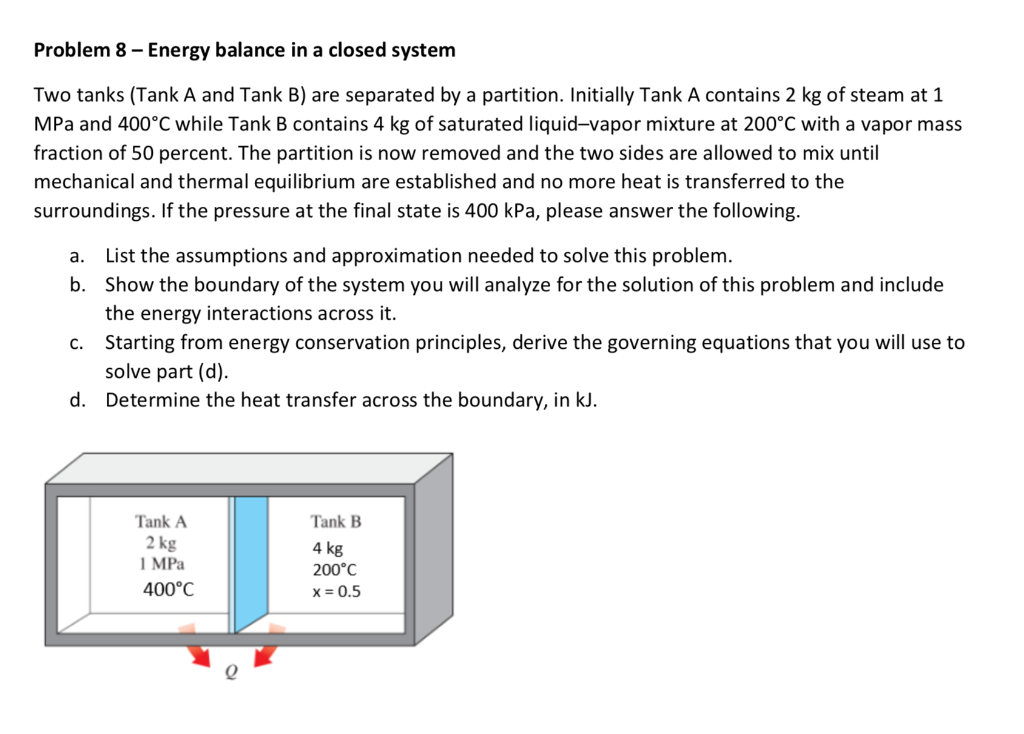
This topic presents a review of energy balance and its applications in a closed system. Therefore the balance becomes. Chemical Engineering Aptitude Test on Application of the Energy Balance to Closed Systems.
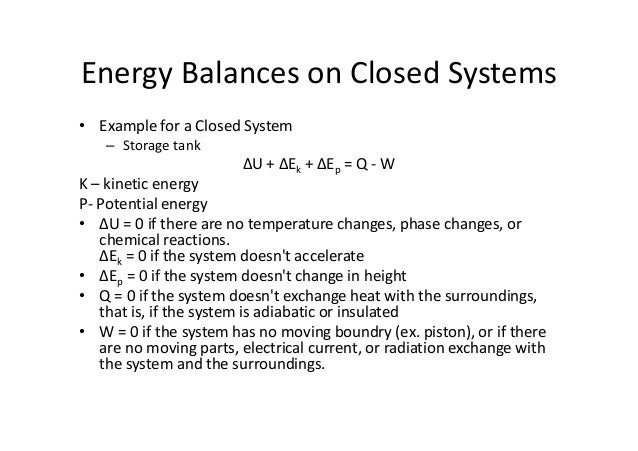
Module 1Energy Analysis of Closed Systems. Although no mass crosses the boundaries energy input 0 and energy output 0 since energy can be transferred across the boundary. Energy Balance for Closed system Energy balance Equation Example Problems.
ΔUQW or Δuqw per unit mass where. If the velocity varies between inlet and outlet the kinetic energy varies.
U the change in internal energy of this closed system Q heat transferred.
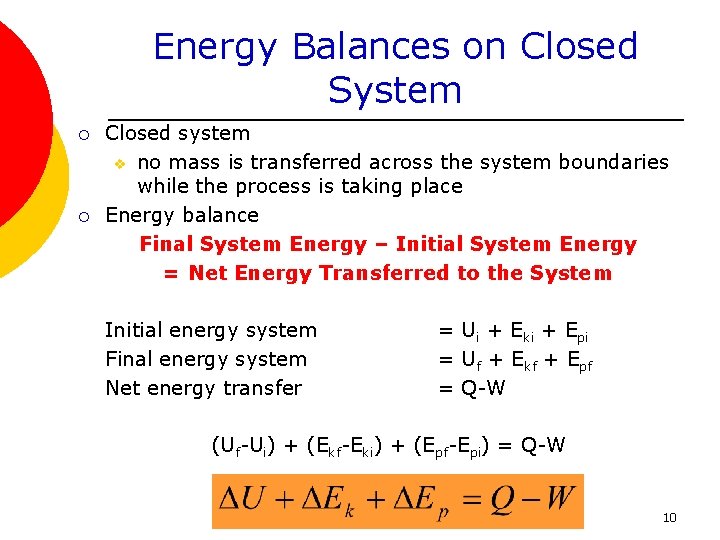
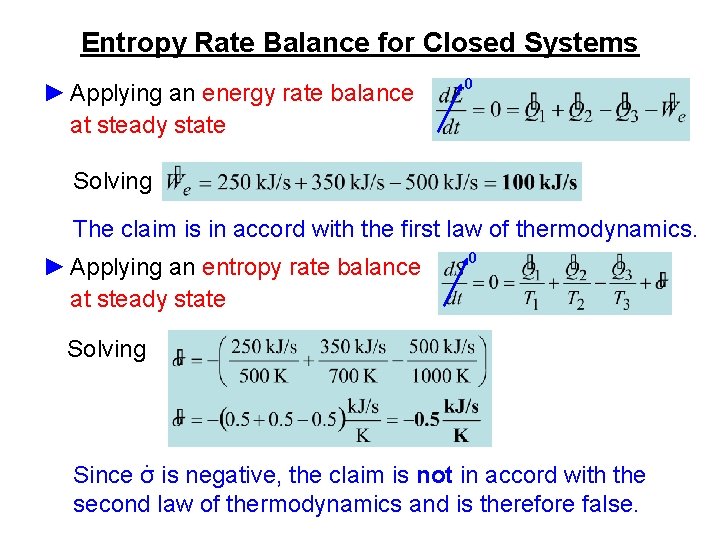
ME 200 L6. UQW or uqw per unit mass where. U the change in internal energy of this closed system Q heat transferred. An energy balance describes the relationship between molecular potential and kinetic energy fluid potential energy fluid kinetic energy heat and work. ΔU the change in internal energy of this closed system. A Matter in the system is constant b Energy in the system is constant c Neither energy nor matter is constant d Both of energy and matter are constant. When a closed system undergoes a process from state 1 to state 2 its energy and entropy balances are. Between a system and its surroundings the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings. ME 200 L6.
ΔU the change in internal energy of this closed system. When a closed system undergoes a process from state 1 to state 2 its energy and entropy balances are. A Matter in the system is constant b Energy in the system is constant c Neither energy nor matter is constant d Both of energy and matter are constant. Therefore the balance becomes. Between a system and its surroundings the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings. The general energy balance or the 1 st Law of thermodynamics for closed systems can be expressed as. Module 1Energy Analysis of Closed Systems.




















Post a Comment for "Closed System Energy Balance"